All lubricants consist of a base oil. Normally, lubricants consist of 90% base oil and 10% additives. The American Petroleum Institute’s (API) has categorized base oils into five groups which are specified by the saturate level, sulfur level, and viscosity index.
Saturate Level
Saturates are a type of molecule commonly found in base oil. They are naturally present in base oil but during the refining process higher levels of saturates are obtained. If the level of saturates is higher, the molecular bond of the oil is stronger. This will increase the resistance to breakdown and oxidation or the loss of viscosity.
Sulfur Level
Sulfur is a natural inorganic element occurring in crude oil. Because it reacts with oxygen it can be harmful to the performance of oil. It can also be damaging to exhaust after treatment devices. Besides these negative aspects of sulfur there are also some positive aspects. Sulfur can be an effective antioxidant which improves the oxidative stability. When the content of sulphur is lower, the purity is better which decreases the probability of corrosion and oxidation.
Viscosity Index
The Viscosity Index refers to the changes in viscosity compared to the temperature of the oil. The viscosity is measured at 40 °C and 100 °C. When the viscosity index is high, the changes are smaller with differences in temperature. All oils increase in viscosity when the temperature decreases and decrease in viscosity when temperatures increase.
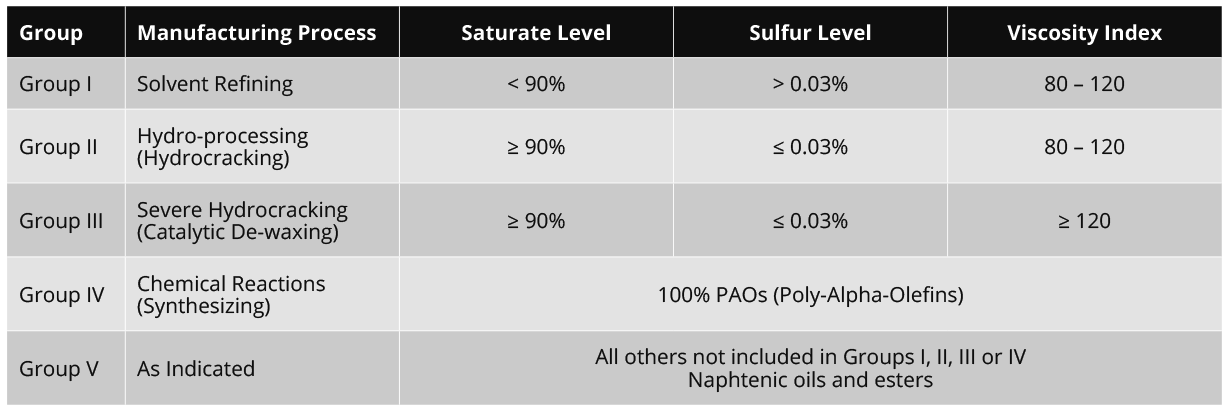
Group I, II, and III are derived from crude oil (mineral oil), Group IV is a fully synthetic oil, and Group V is for all base oils that are not included in one of the other groups.
Group I
Group I oils are solvent-refined, which is a simpler refining process, making them the least refined and therefore also the cheapest base oils available. Solvent-refined oils consist commonly of a mix of different hydrocarbon molecules which cannot be distinguished in the refining process. This results in an oil with irregular molecules causing increased friction within the oil. Group I oils are therefore used most often in less demanding applications.
Group II
Group II base oils undergo hydrocracking which is a more complicated process than the process for Group I oils. Hydrocracking is a process that breaks down large hydrocarbon molecules into smaller ones. The hydrocarbon molecules of these oils are saturated, giving them better antioxidation properties. Group II oils are priced closely to Group I oils.
Group III
Group III oils undergo an even longer process than Group II oils. The process, called severe hydrocracking, is also more intense. More pressure and heat is applied during the refinery process. This results in a purer base oil with a higher quality. Even though Group III oils are derived from crude oil, they are sometimes described as synthesized hydrocarbons.

Group IV
Group IV base oils are polyalphaolefins. These are not extracted but made from small uniform molecules. This is also the biggest advantage of PAOs because they can be completely tailored to have a structure with predictable properties. They are very suitable for use in extreme cold or extreme hot conditions.
Group V
Group V oils consist of any type of base oil other than mentioned in the previously defined groups. If it is a synthetic oil and it is not PAO it is a group V base oil. They include, among others, of naphthenic oils and esters. Usually Group V oils are not used as a base oil but as an additive to other base oils.


